Gasses That Cause Acid Rain
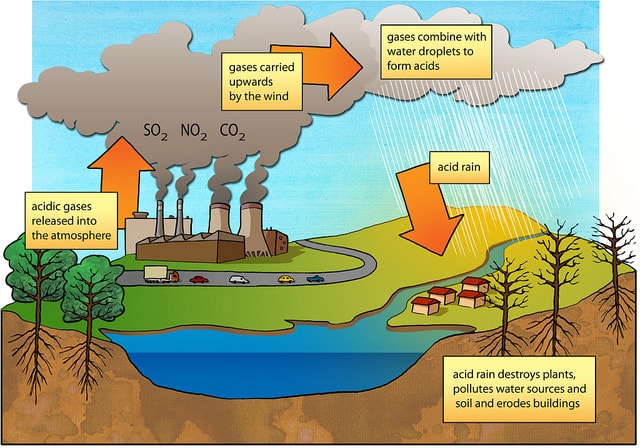
Web Acid rain occurs when sulfur dioxide and nitrogen oxide gases react in the atmosphere with water oxygen and other chemicals to form various acidic compounds such as sulfuric acid and nitric acid. These substances can rise very high into the atmosphere where they mix and react with water oxygen and other chemicals to form more acidic pollutants known as acid rain.
Acid rain causing gases.
. SO2 sulfur dioxide will make sulfuric or sulfurous acid. Power plants release the majority of sulfur dioxide and much of the nitrogen oxides when they burn fossil fuels such as coal to produce electricity. Acid rain caused by burning coal and oil products to fuel our societys needs has damaged trees in many areas of North America.
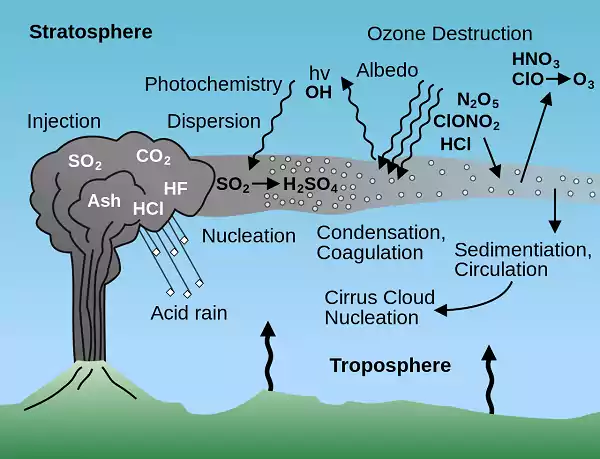
The main natural causal agent for acid rain is volcanic emissions. If the pH-level changes due to acid rain the plants may no longer be able to grow. Volcanoes emit acid-producing gases mainly sulfur to create higher than normal amounts of acid rain or any other form of precipitation such as fog or snow to an extent of affecting vegetation cover and health of residents within the surrounding.
These include carbon dioxide nitric oxide nitrogen dioxide and sulfur dioxide. Web Human activities are the main cause of acid rain. Web The major solution to acid rain is to reduce and curb the emission of gases that causes acid in the rain.
Web The gas most responsible for the acid rain effect on plants and water systems is sulfur dioxide. What gases cause acid rain GCSE. This is only possible by controlling and regulating the sources of NO2 and SO2.
NOx oxides of nitrogen from combustion will make nitric acid. Several gases resulting from fossil fuel combustion can form acidic compounds in rain. The most common cause of acid rain is the pollution caused by fossil fuels.
Where pure water has a text pH of 7 acid rain has a text pH of about 50-55 and sometimes it could be 4 where there are a lot of industries and cars. Web Best Answer. Web Acid rain isnt pure acid falling from the sky.
The fact is as long as fossil fuel is in use the problem of acid rain will continue. Web The gas most responsible for the acid rain effect on plants and water systems is sulfur dioxide. Web Acid rain is caused primarily by sulfur dioxide SO2 and nitrogen oxide NOx emissions.
Web Acid rain is caused by many other pollutants as well which have varying effects on waters fish and plant life. Many plants need a stable pH-level in order to grow. Web Acid rain is a pollution problem caused by the release of acidic gases into the atmosphere.
They also dirty the surfaces of buildings and other structures. One of the primary causes of acid rain is carbon dioxide a toxic gas that has been responsible for global warming and climate change. A higher level of acidity in the soil leads to a change in the growth behavior of plants.
Over the past few decades humans have released so many different chemicals into the air that they have changed the mix of gases in the atmosphere. When these gases react with the moisture in the atmosphere sulfuric and nitric acids form. A study showed that in China acid rain may have contributed to the deadly.
It contributes to pollution in a variety of ways including. Web Causes of Acid Rain. Web The world is changing for the worse.
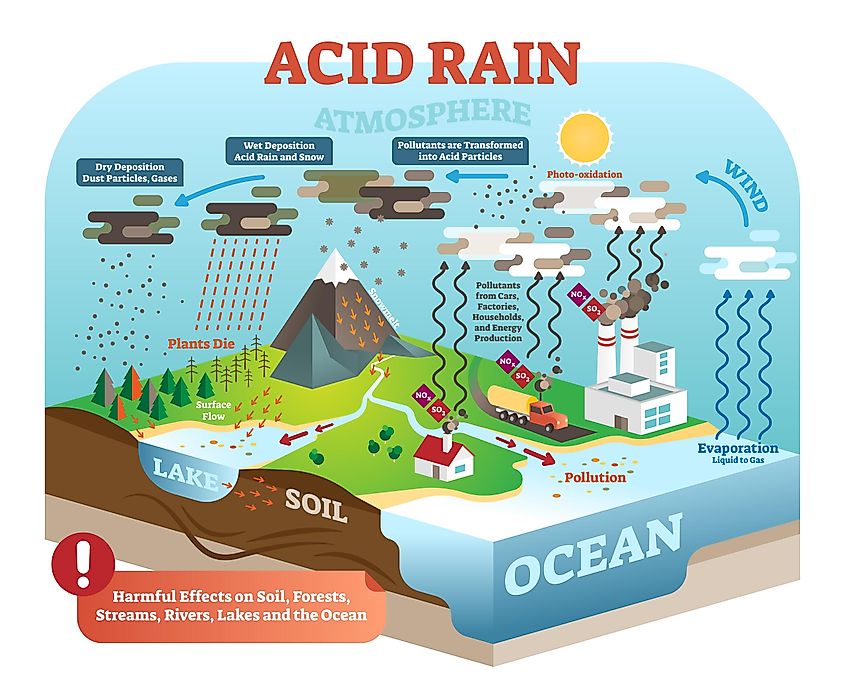
Wind may transport these acid particles over long distances before they come down as wet or dry deposition. The causes of acid rain are Sulphur and Nitrogen particles which get mixed with the wet components of rainSulphur and Nitrogen particles which get mixed with water are found in two ways either man-made ie as the emissions that are given out from industries or by natural causes like lightning strike in the atmosphere releasing nitrogen. However certain pollutants released into the atmosphere.
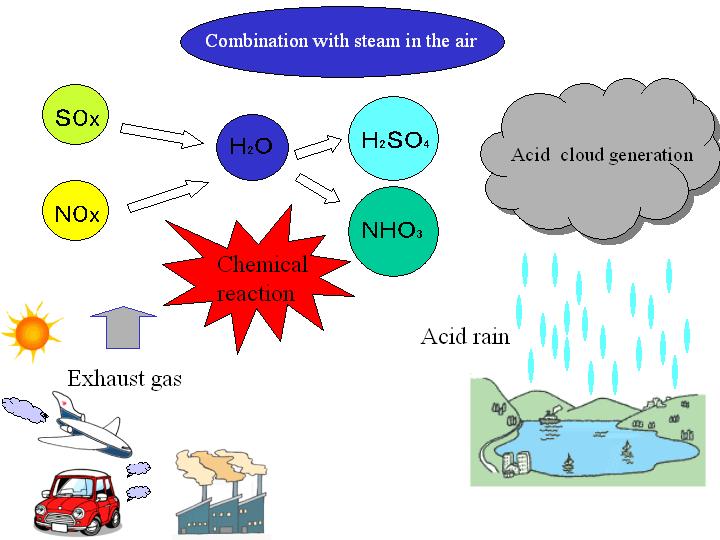
As fossil fuels form a gas when burned some of the metals from them react with water vapor in the air creating the acid. These produce sulfur dioxide a gas with a sharp choking smell when the fuel is burned. Web Acid rain is caused by a chemical reaction that begins when compounds like sulfur dioxide and nitrogen oxides are released into the air.
Web Sulfur dioxide and acid rain. Web Acid rain leads to a change in these living conditions since it makes the soil more acid. All rain is weakly acidic due to dissolved carbon dioxide.
These gases are introduced into the atmosphere from natural resources volcanoes and man. The acidic particles corrode metal and cause paint and stone to deteriorate more quickly. When sulfur dioxide dissolves in water droplets in clouds it makes the.
The gas most responsible for the acid rain effect on plants and water systems. Fossil fuels naturally contain sulfur compounds. Web When acid rain and dry acidic particles fall to earth the nitric and sulfuric acid that make the particles acidic can land on statues buildings and other manmade structures and damage their surfaces.
Rather rainfall or atmospheric moisture mixed with elements and gases cause the moisture to become more acidic than normal. Two gases will contribute to acid rain. Acid Rain Caused By Carbon Dioxide.
Primary Causes Of Acid Rain Earth Eclipse
Acid Rain A Regional Environment Challenge Follow Green Living
Comments
Post a Comment